As previously stated, in mammalian blood, melatonin levels exhibit a low rate rhythm during the day and high levels at night [162-166]. Since this is common to all mammals, regardless of their pattern of locomotor activity, the melatonin has been defined as the hormone of darkness or chemical expression of the dark [167]. This diurnal pattern of fluctuation in melatonin levels in animals is perturbed upon exposure to light during the night [168-170], while in constant darkness the rhythm persists, ie, the rhythm is circadian.
Considering the nature of the melatonin release rate in the blood in mammals, there was a strong interest in similar variations potentially existing in plants and whether melatonin was involved in photoperiodism in these species. Using a herb (Chenopodium rubrum, syn, Oxybasis rubra, red goosefoot, and other common names) that commonly grows in temperate climates in many areas of the world, Kolar et al. [171] found that nocturnal melatonin levels in this species exceeded those measured in plants harvested during the day. Thus, in this plant, as in mammals, the melatonin rhythm could provide information regarding the length of the night.
In contrast to these observations, Tan et al. [172] in a study on water hyacinth (crassipes Eichornia (Mart) Solms) found that melatonin levels dictate a rhythm, but peak levels occurred towards the end of the light period, rather than at night. Thus, melatonin clearly does not serve as a messenger of the dark in this plant species. Additionally, daytime melatonin levels were higher when plants were grown under sunlight (10,000-15,000 W / cm2) than when plants grown indoors with artificial light (400-450 W / cm2). In addition to the observed melatonin rhythm, there was a similar N1-N2-acetyl-formyl-5-methoxykinuramine (AFMK) cycle; this product peaked shortly after melatonin. Since AFMK is a metabolite of melatonin, present in free radical scavenging, it has been hypothesized that melatonin in water hyacinth as well as in animals functions as a radical scavenger [173-178]; this explains the increase in AFMK shortly after the melatonin peak. That melatonin functions as a free radical scavenger in this plant is also supported by the finding that melatonin concentrations were much higher in plants grown under sunlight than under indoor light. The intensity of external light causes highly elevated photosynthetic activity, a process that generates free radicals [179]. Consequently, the increase in free radicals presumably initiates a compensatory increase in melatonin to protect the plant from oxidative stress.
A third rhythmic pattern of melatonin was described by Tal et al. [180] in a green macroalga. The genus of the alga is the Ulva, but the species could not be identified [181,182]. Even when this species was grown as a free floating culture below photoperiod and constant water levels, it showed a semi-lunar rhythm of melatonin concentrations in a way related to the spring tides. These researchers hypothesized that the elevated melatonin levels at the time of low tides were meant to protect the alga from an increase in oxidative stress that normally occurs due to low water levels. During this time, the algae are subjected to high temperatures, drying and changes in salinity, all of which cause stress. Thus, like Tan et al. [172] in the water hyacinth, Tal et al. [180] found a endogenous increase in melatonin which occurs as a consequence of natural environmental stress.
The cherries (Prunus cerasus) represent the first fruit in which melatonin levels have been extensively studied [183]. The two varieties tested, namely, Montmorency and Balaton, markedly contain different concentrations of melatonin [13.4 μg / g FW (fresh weight) and 2.1 μg / g FW respectively]. Considering that melatonin is a sleep-promoting agent [184,185], the authors of this work suggested that the consumption of cherries or especially the intake of concentrated cherry juice (which contains much higher levels of melatonin than the cherries themselves ) can improve sleep quality. Subsequent publications relating to these data suggest that products containing these fruits may be useful for improve sleep quality, particularly in the elderly [186-190].
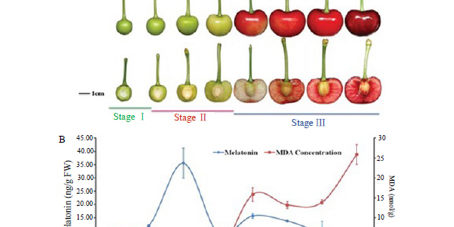
Finally, in at least two cherry fruit varieties, melatonin levels vary over a 24 hour period and also with fruit development [190]. In the cherries "Hongdeng" (Prunus avium L. cv. Hongdeng) and "Ranieri" (Prunus avium L. cv. Ranieri) the trend in the 24h of melatonin showed two peaks. The night peak occurred around 05:00 h and during the day the peak was evident in the late afternoon, corresponding to the highest temperature and light intensity. The highest levels of melatonin in the “Hongdeng” cherry were around 7.7 μg / g FW while in the “Ranieri” cherries the values approached 20 μg / g FW. Measurements over a period of 24h were made on fruit during development phase 1 (shortly after the flower falls with rapid fruit expansion and rapid growth of the pit and endosperm).
For both “Hongdeng” and “Ranieri” cherries, the levels of melatonin vary greatly with the stage of development of the fruit. Melatonin levels were highest in stage 2 of development (period of embryonic development and endocarp lignification). Peak levels of melatonin at this stage were again higher in "Rainier" cherries than in "Hongdeng" cherries (about 125 versus 36 μg / g FW).
During phase 3 of fruit development (fruit growth and skin and fruit coloring) the levels of melatonin remained low and were inversely correlated with the levels of peroxidized lipids in the fruit. Due to the inverse relationship of melatonin-lipid peroxidation (in phase 3) and the very high levels during phase 2 (where free radical generation is highest), Zhao et al. [190] concluded that the melatonin represents an antioxidant in the cherry fruit. A similar relationship was found between apple ripeness levels (Malus domestica Borkh. Cv. Red) and melatonin levels by the research group of Lei and colleagues [191]. As in the cherry tree, higher melatonin levels co-existed with the periods of faster growth and increased respiratory rate in the apple, times when free radical generation is at its peak.
Again, there was also an inverse association between melatonin concentrations and the amount of peroxidized lipids. In addition to documenting that melatonin concentration changes drastically during apple development, this is the first study identifying melatonin in this fruit. Clearly, there are variations in melatonin concentrations in mature plants as well as animals [180].
As summarized below, there are a variety of factors that influence the synthetic activity of melatonin in plants and with the observed rhythms, for example, the cyclic variations noted by Kolar et al. [171] and Tan and colleagues [172], are probably related to environmental changes in the 24 hour period that improve the number of free radicals that are formed while those reported by Zhao and collaborators [190] and Lei et al. [191] are probably driven by intrinsic metabolic processes which also induced a generation of free radicals.
Abstract of the thesis "Melatonin in the plant world (Phytomelatonin): therapeutic properties and future prospects" by Dr. Giorgio Guerrini
For further information, please refer to the bibliography page.
The information contained on the Site, in the various articles, in the contents, in any responses to comments, are for informational purposes only and in no way have the claim or purpose of replacing the opinion of the doctor and / or specialist, of others. health professionals or professionals in the sector who must in any case be contacted and consulted for the formulation of a diagnosis or the indication of a possible correct therapeutic and / or dietary and / or food supplement program, and more generally for the comparison on this information. In no case will the Site, the Publisher who manages it, the authors of the articles and / or contents and / or comments and / or blogs, or other subjects connected to the Site, be responsible for any damage, even if only hypothetically connected. the use of the contents and / or information present on the Site. The Site assumes no responsibility for the misuse that users may make of the information contained in the Site.